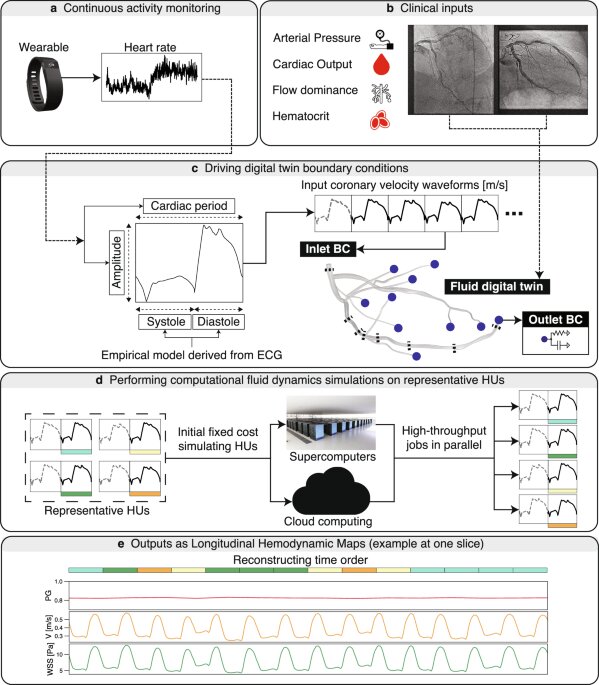
In a groundbreaking endeavor, a novel framework called the Longitudinal Hemodynamic Mapping Framework (LHMF) has been established, aiming to revolutionize cardiovascular health monitoring through wearable technology. This comprehensive framework captures three-dimensional longitudinal hemodynamic behavior and uses this data to develop coronary digital twins. This significant technology advancement integrates various biomedical data streams to create a detailed representation of an individual’s cardiac health over time.
The framework begins by acquiring continuous heart rate signals from wearable devices. This data is crucial as it personalizes the inlet coronary velocity waveforms, adjusting them in real-time based on the patient’s continuously changing physiological states. Accompanying Fig. 1a of the study illustrates this initial step. Image-based anatomical models of the coronary system are then reconstructed using coronary angiograms, with expert cardiologists evaluating the reconstruction quality (Fig. 1b). Alongside imaging, clinical measurements such as arterial pressure, cardiac output, and hematocrit levels are also recorded (Fig. 1c) to fine-tune the simulations to reflect each patient’s unique cardiovascular conditions.
Advanced computational techniques are employed to simulate these personalized boundary conditions. Using pulsatile velocity waveforms and Windkessel models, the framework simulates the patient’s microvascular resistance. Long sequences of inlet coronary velocity waveforms are broken down into representative sets for parallel computations, significantly streamlining the process (Fig. 1d). The simulations are executed using HARVEY, a state-of-the-art fluid dynamics solver, to provide detailed 3D blood flow simulations (Fig. 1e).
Leveraging Wearable Data for Real-Time Cardiac Monitoring
Harnessing data from wearable devices, the LHMF aims to personalize and dynamically update coronary digital twin models. Unlike traditional methods that rely on static snapshots of cardiac cycles, this framework utilizes continuous heart rate and electrocardiography (ECG) data to adapt and tune the coronary velocity waveforms. This real-time data adaptation mirrors natural physiological changes, such as fluctuations in heart rate due to different activity levels. Employing multimodal wearable data enhances the accuracy of data inputs, thus refining simulations to accurately reflect varying physical states.
Deconstructing Temporal Dependence to Enable Parallelized Simulations
A standout feature of the LHMF is its ability to deconstruct the sequential order of heartbeats, enabling parallelized simulations to capture 3D longitudinal hemodynamics effectively. Inspired by DNA genome shotgun sequencing, this method breaks down long sequences of heartbeats into hemodynamic units (HUs), which are simulated individually (Fig. 1d). These HUs include both the heartbeat of interest and preceding cardiac cycles to ensure temporal convergence. The reassembly of the simulated HUs allows for a comprehensive longitudinal hemodynamic behavior capture (Fig. 1e).
Ensuring Temporal Convergence Across Varying Activity States
Temporal convergence is essential for accurate hemodynamic simulations. The LHMF undergoes rigorous testing to determine the number of preceding cardiac cycles required for temporal convergence across various heart rates. A study on data spanning 6 weeks and encompassing heart rates from resting to exercise states confirmed that accurate simulations generally needed minimal pre-flow cycles. Critical hemodynamic metrics like resting pressure gradient, blood velocity, and wall shear stress achieved convergence swiftly.
Validation and Practical Application
The LHMF was meticulously validated against a ground-truth explicit 3D CFD simulation covering 750 heartbeats. This validation ensured the framework’s ability to replicate real-world cardiac behavior with high accuracy. By aggregating data over multiple anatomical positions and metrics, the LHMF demonstrated over 99.995% accuracy, establishing its reliability for practical applications.
In addressing scalability, the refined LHMF, known as LHMF C, reduces the number of necessary simulations by clustering hemodynamically similar HUs. This clustering minimizes redundant computational efforts, significantly reducing the time and resources required for generating longitudinal hemodynamics. This efficiency makes the framework feasible for extensive longitudinal studies, where millions of heartbeats are analyzed.
Deploying Across Diverse Computing Platforms
The expanded utility of the LHMF and LHMF C is evident in their deployment across various computing platforms, including traditional clusters and modern cloud-based systems. The adaptability of the framework to different computational environments ensures swift and efficient processing of data, which is vital for practical clinical implementation.
The Future of Coronary Health Monitoring
The development of LHMs represents a significant leap in cardiac health monitoring. These maps provide detailed spatial and temporal visualization of hemodynamic metrics, highlighting areas susceptible to disease over extended periods. For instance, in regions where low wall shear stress persists, there is a higher propensity for atherosclerotic plaque development, indicating potential disease progression.
By leveraging continuous wearable data, the LHMF framework offers an unprecedented level of precision in monitoring and predicting cardiovascular health. This advancement not only enhances patient-specific treatment plans but also paves the way for proactive healthcare interventions, ultimately leading to improved outcomes in coronary health management.
In summary, the LHMF and its refined iteration, LHMF C, herald a new era in cardiovascular diagnostics, combining cutting-edge wearable technology with sophisticated computational techniques to deliver a dynamic and comprehensive view of heart health.